Ole G. Mouritsena* , Lars Duelundb, Ghislaine Callejaa, and Michael Bom Frøsta – Nordic Food Lab & Taste for Life, Section for Design and Consumer Behaviour, Department of Food Science, University of Copenhagen, Rolighedsvej 26, DK-1958 Frederiksberg C, Denmark
Lars Duelundb – MEMPHYS, University of Southern Denmark, 55 Campusvej, DK-5230 Odense M, Denmark
Keywords:
Fermentation
Fish sauce
Insects
Grasshopper
Wax moth
Pheasant
Peas
Umami
Glutamate
Aspartate: Amino acids
Nucleotides
http://dx.doi.org/10.1016/j.ijgfs.2017.05.002
Received 2 January 2017; Accepted 11 May 2017
⁎ Corresponding author.
E-mail address: ole.mouritsen@food.ku.dk (O.G. Mouritsen).
Fermentation of dairy products, legumes, fish, shellfish, molluscs, and meat are considered to lead to some of the most flavourful products in cuisines across the world. The deliciousness of these fermented products is to a large extent due to compounds like free amino acids and free nucleotides, formed during fermentation, which impart umami taste, often in a synergistic fashion. We have prepared fermented fish sauces based on mackerel, using procedures as in the ancient Roman cuisine, and used similar techniques to produce experimental fermented sauces from insects (moths and grasshoppers), game (pheasant), and pulses (peas). In some cases, the fermentation has been facilitated by fungal inoculation based on a Japanese koji mother. We have performed chemical analysis of these experimental fermentation products, together with a comparative analysis of a series of commercial fish sauces, with particular focus on free amino acids and free nucleotides in order to assess the umami potential of the various products. Whereas all of the 21 different investigated sauces are found to have high amounts of glutamate and aspartate, no significant amounts of free nucleotides are found in any of the samples. The investigated sauces were characterized by quantitative sensory evaluation. Although high in glutamate, umami synergy is not expected to play any significant role for the flavour of these fish, insect, game, and pea sauces. The sensory analysis shows a fairly good prediction of sensory properties from the chemical characterization of the sauces. However, the relationship between glutamate/aspartate concentration and intensity of umami taste is not simple. It demonstrates that in the complex solutions that constitute these sauces, there may be other perceptions that interfere with the main umami-tasting compounds.
Abbreviations: AA, amino acid; AAS, atomic absorption spectroscopy; AMP, adenosine-5′-monophosphate (adenylate); ANOVA, analysis of variance; A–PLSR, ANOVA–partial least squares regression; GMP, guanosine-5′-monophosphate (guanylate); HPLC-MS, high-pressure liquid chromatography mass spectrometry; IMP, inosine-5′-monophosphate (inosinate); MSA, monosodium aspartate (aspartate); MSG, monosodium glutamate (glutamate); PLSR, partial least squares regression; UMP, uridine-5′-monophosphate International Journal of Gastronomy and Food Science 9 (2017) 16–28
Available online 17 May 2017
1878-450X/ © 2017 Elsevier B.V.
Some of the most flavourful and highly appreciated foodstuffs in many cultures around the world owe their deliciousness to taste compounds developed during fermentation and ageing processes, either by use of yeast, bacteria, moulds, and enzymes or combinations hereof (Mouritsen and Styrbæk, 2014). Fermented products are often considered an integral part of a food culture (Katz, 2012). Well-known examples include fermented milk (cheeses, sour-milk products), fermented beans (soy sauce, miso, nattō, douchi, furu), fermented grapes, fruit, berries, and grains (wine, beer, cider, brandy, bread), fermented vegetables (sauerkraut, kimchi, tsukemono), fermented leaves (tea), fermented fish (katsuobushi, fish- and shellfish sauces, Swedish surströmming), and fermented meats (sausages, hams). In most cases, the liking of fermented foodstuff requires adaptation and in some cases it is an acquired taste.
In the classical culinary triangle proposed by Lévy-Strauss (1983), the raw foodstuff can be changed either by cooking (temperature) into edible foodstuff or by microbiological degradation into rotten and inedible stuff. ‘The ‘cooked’ and ‘the rotten’ then represent culture and nature, respectively. However, the borderline between ‘the cooked’ (prepared) and ‘the rotten’ is not sharp, and different cultures as well as cultures at different times have varying perceptions regarding what is considered edible and what is not. Moreover this ‘culinary borderland’ is a flexibly boundary that is constantly subject to negotiation and reinterpretation (Højlund et al., 2014). Manipulating and mastering fermentation processes is a way to navigate this borderland. As pointed out by Vilgis (2013), by considering fermentation and long-time cooking as a desirably way to degrade proteins and carbohydrates in prepared food, in much the same way as spontaneous degradation in undesirable putrefaction, the borderland between ‘the cooked’ and ‘the rotten’ tends to vanish.
Fermentation releases a range of flavourful taste compounds and aroma substances. Often a repulsive smell can overpower the desire to taste the fermented foodstuff. In many cases the fermentation processes produce a bounty of nutritional elements that are more easily digestible and provide for enhanced bioavailability in the gastrointestinal system. At the same time, properly conducted fermentation leads to preservation and prolonged lifetime of the prepared foodstuff, e.g., via dehydration or production of alcohol and lactic acid that suppress undesired fungal and bacterial growth as well as putrefaction.
In some cases, enzymatically facilitated low-temperature Maillard reactions during the fermentation may release additional flavourful browning products (Nursten, 2005), as is well known, e.g., from soy sauce and black garlic, although the process at low temperatures takes very long time.
The success of a fermentation process applied to foodstuffs is from the point of view of microbiology a delicate balance involving the risks of cross-contamination, wild fermentation outcompeting culturing, and growth of unwanted and potentially hazardous, poisonous fungal and bacterial cultures (Katz, 2012). Cultured fermentation typically requires good measures of cleanliness, temperature control, possible sterilization, and the use of starter cultures. High levels of salt (sodium chloride) and low pH are the most important parameters suppressing the growth of undesired microorganisms and leading way to the degradation of proteins, carbohydrates and nucleic acids.
There is a significant difference in the approaches used to ferment plant matter and meat from animals (Katz, 2012). Plants and foodstuff derived from plants contain large amounts of carbohydrates and sugars that are rapidly turned over by lactic bacteria and yeasts, producing alcohol and lactic acid, which suppress the growth of potentially hazardous bacteria and fungi. In contrast, meat and fish contain very little carbohydrates. Milk as an animal derived product is an exception that is readily susceptible to lactic fermentation. Fermentation of meat can be a risky business due to pathogenic microorganisms that may produce dangerous toxins, in particular when these organisms get access to the interior of the meat that in the living state is sterile.
Fermentation of meat therefore usually involves pre-treatment by salt and application of drying or smoking techniques. In the case of fish, halotolerant visceral enzymes along with various aerobic and anaerobic salt-loving bacteria are able to ferment the pre-treated flesh and prevent putrefying bacteria to get into action. In this case, it is basically a principle of establishing conditions that selects the desirable bacterial and fungal cultures.
Whereas there is a substantial literature about fermentation of plants and plant materials (Katz, 2012), meat (Ockerman and Basu, 2008), as well as fish (Lopetcharat et al., 2007), there is very little published in the scientific literature about insects and in particular fermented insects as foodstuff (van Huis et al., 2014). In the present paper we describe an experimental attempt to produce a gastronomically acceptable fermented sauce from grasshoppers and wax moth larvae and deliver some of the first chemical analysis of insects sauces, combined with the first detailed sensory analysis of them. In the context of the Western cuisine, the particular combination of fermentation and insects represent an ultimate example of the challenging and less edible corner of Lévy-Strauss’ culinary triangle.
In this paper we shall focus on the flavour of the fermented sauces, in particular from taste components derived from proteins and nucleic acids. Being large molecules, proteins and nucleic acids have no taste of their own since they cannot be detected by the taste receptors. Only when broken down into smaller constituents, in particular free amino acids and free nucleotides, respectively, can they stimulate appropriate taste receptors, such as the umami receptor (Mouritsen and Khandelia, 2012). In certain cases, small peptides can also elicit taste, e.g., kokumi taste by the tripeptide glutathione (Maruyama et al., 2012; Kuroda et al., 2012). During fermentation, the proteins and nucleic acids in the raw materials are subject to degradation, and large amounts of free amino acids and nucleotides can potentially be formed. However, the temporal evolution of the contents of these compounds is often highly complex and may be non-monotonic because the produced free nucleotides and amino acids may be consumed at a later stage in the fermentation process, which is the case in beer production. Similarly, high levels of free inosinate in fresh fish may be diminished by postmortem autolytic decay and vanish almost completely during fermentation of fish sauces leading way to less-well tasting compounds, such as inosine or hypoxanthine (Gill, 1990).
Free glutamate and free nucleotides, in particular inosinate, guanylate, and adenylate, enter a unique synergic relation due to an allosteric action on the T1R1/T1R3 umami receptor (Zhang et al., 2008; Mouritsen and Khandelia, 2012). Being highly non-linear, this synergy implies that small amounts of one component, e.g., inosinate, can enhance the sensory perception of glutamate manifold (Yamaguchi and Ninomiya, 2000). This pairing of food items is hence based on a scientific principle in contrast to the claimed food pairing principle based on chemical identity, a principle that proven to be based on culture rather than a scientific principle (Ahn et al., 2011). The proposal of umami as a fifth basic taste (Ikeda, 2002) was based on the finding of large amounts of free glutamate in the brown algae konbu used for production of the Japanese soup stock dashi (Ninomiya, 1998;
Yoshida, 1998). The synergetic action and the umami-pairing principle in traditional dashi are mediated by inosinate from a fish product, katsuobushi, and in vegetarian shōjin dashi by guanylate from dried shiitake. The umami-pairing principle behind the delicious flavour of dashi is the same that is operative in the well-known combinations of foodstuff in the Western cuisine, e.g., eggs-bacon, cheese-ham, and vegetables-meat (Mouritsen et al., 2012; Mouritsen and Styrbæk, 2014).
Only few types of foodstuffs, most prominently sun ripe tomatoes and nori (from the red macroalga species Pyropia spp.) can alone account for both components in umami synergy, and therefore at least two different ingredients are required. However in many cases, due to the non-linear synergetic action, one of the ingredients can be added in small amounts, e.g., a drop of fish sauce or soy sauce, a bit of anchovy paste, a stench of tomato paste, a crumble of Parmesan, or bit of blueskim cheese.
In principle one would have thought that fermented fish sauces could provide both glutamate and inosinate, because fresh fish like mackerel, anchovies, and sardines contain large amount of inosinate and the subsequent fermentation might produce plenty of glutamate.
However, inosinate is quickly broken down enzymatically soon after the fish is dead (Gill, 1990), and very special preparation techniques, e.g., as perfected in the production of katsuobushi (skipjack tuna) and niboshi (sardines), have to be invoked in order to conserve the inosinate. Alternatively the fish could be cooked, dried, and marinated immediately after catching (Maga, 1983), e.g., for producing anchovy paste. In both cases, however, the proteolytic enzymes that could produce glutamate during subsequent fermentation would be inactivated, rendering a product with only little free glutamate. Hence it appears that fermented fish sauces would foremost be glutamate providers.
Whereas there is a wide range of fermented molluscs (e.g., fermented squid) and shellfish (e.g., shrimp paste and original oyster sauce) sauces in Southeast Asia, fish sauces of various origins are undoubtedly some of the most prevalent popular fermented products in the world cuisine, not least in Asia (Lapsongphon et al., 2015). There is rich cultural history behind fermented fish and shellfish products and how they have developed into modern condiments like soy sauce, English sauce, and ketchup products that no longer contain fish (Mouritsen and Styrbæk, 2014). Although these modern products can contain large contents of free glutamate, traditional fish sauces generally dwarf them.
Seafood, it be fish, shellfish, molluscs, or seaweeds, are known to be good sources of umami-tasting compounds (Komata, 1990) and therefore also a good starting point for producing fermented sauces with a very high potential for umami. Fish is a natural and readily available source for producing fermented sauces (Lopetcharat et al., 2007) because fish contain aggressive endogenous enzymes, e.g., in viscera, such as proteases and nucleases that digest proteins and nucleic acids, respectively. The most important contributor to umami in fish sauces is free glutamate. The content of free glutamate can be very large, close to 1400 mg per 100 g in Japanese ishiri and the Vietnamese nuoc mam tom cha, or a little more than in soy sauce (Yoshida, 1998). In contrast, fish sauces seem to contain very little free nucleotides (Park et al., 2001, Funatsu, 2000a; b). Fish sauces are generally produced with high levels of salt, all the way up to 25%, which is considerably more than the 14–18% found in soy sauce (Yoshida, 1998). The combination of umami compounds and salt works synergistically to enhance the saltiness of fish sauce. The less salty fish sauces typically lead to more flavourful products, since the salt tends to limit the enzymatic activity (Lopetcharat et al., 2007). Similarly, the addition of soy sauce to different foods has been demonstrated to be able to reduce the total salt in some prepared foods with 17–50% without noticeable loss of saltiness (Goh et al., 2011; Kremer et al., 2009).
The outline of the remaining part of the paper is as follows. Since our experimentation with fermented sauces naturally writes itself into the grand tradition of ancient fish sauces, we first briefly review and revisit the tradition of the Roman fish sauce garum and offer a few comments about modern East Asian fish sauces for the sake of later comparison. We then describe our experiments with fermenting sauces of fish, insects, game, and peas, leaving technical details, methods of sensory evaluation, and recipes to the “Materials and Methods” section.
In the first “Result” section we present the result of the chemical analyses of the various fermented sauces, including a series of commercial fish sauces. In the second “Result” section, we describe the results of the sensory evaluation of the various sauces.
Penultimately, the results obtained are discussed in “Discussion” and some comments are offered regarding the gastronomic potential of the fermented sauces, specifically with respect to eliciting umami taste and how this correlates with the chemical composition. The paper is closed by “Conclusion” and “Discussion” sections.
Fermented sauces and pastes made from fish, shellfish, and molluscs, possibly more than 300 different types, are common and widespread throughout most of Southeast Asia. They are known by a number of different names, some of which are yu-lu (China), nuoc mam tom cha (Vietnam), nam-pla (Thailand), teuk trei (Cambodia), nam-pa (Laos), patis (Philippines), bakasang (Indonesia), ngan-pyaye (Myanmar), budu (Malaysia), and ishiri or shottsuru (Japan). These sauces are generally made according to the same basic method, involving the salting and fermenting of either whole fish and shellfish or parts thereof, including blood and innards. Fermentation is mediated by halotolerant enzymes found in the fish and shell fish as well as various salt-tolerating microorganisms, releasing an abundance of flavour compounds (Dougan and Howard, 1975; McIver et al., 1982; Funatsu et al., 2004; Lopetcharat et al., 2007). Some sauces are made with fresh fish, while others use dried fish. Anchovy is one of the most commonly used species. Sometimes the fish is combined with enzymatic active material from other species, e.g., Japanese ishiri is made from fermented sardines, other small fish, and the innards from squid.
Modern Thai fish sauce is fermented for up to 18 months, much longer than the Roman garum of antiquity, and it also has a considerably higher salt content. Fish sauces have come to be associated with some very traditional dishes. In Korea, chokkaru, made from fermented salted fish, shrimp, and molluscs, is added to that country ׳s best known dish, kimchi, which is fermented Napa cabbage sometimes mixed with other vegetables. Similarly, in Japan, ika no shiokara consists of small squid fermented using the enzymes from their own innards.
The Romans were very passionate about a salty, fermented fish sauce, called garum, and later also liquamen. It was used both as a salt seasoning and to add deliciousness—what we would now call umami taste. Classical Roman works on the culinary arts suggest that garum could be incorporated into just about any dish, even sweet soufflés.
Probably, however, it was most often mixed with something else, for example, with wine vinegar (oxygarum), with honey (meligarum), and with wine (oenogarum).
The etymology of the word garum is a bit of a mystery; it is derived from a fish known to the ancient Greeks as garos; Pliny the Elder includes it in his list of the fish found in the oceans, but without any further description. The sauce was made on the Aegean islands at least as early as the fifth century BCE, and eventually its production spread to many of the fishing towns all around the Mediterranean and the Black Sea region (Bekker-Nielsen, 2005). No sources describe exactly how it was made, only that it was made from fish and that it smelled horrible. While early writers sometimes characterize it as putrid, in other writings they express an enthusiastic fondness for the sauce, referring to its “very exquisite nature.”
It appears that the term ‘garum’ applied only to the brownish liquid that seeped out when small fish and fish intestines, especially those from mackerel and tuna, were salted, crushed, and fermented. Fish sauces were traditionally prepared by mixing salt (up to 25 w/w %) and whole fish and placing the mixture in a warm place for 3–12 months.
The most valuable garum was made from the blood and salted innards of fresh mackerels that were fermented for two months. A lower quality sauce, muria, was made from the briny liquid drawn out when tuna was preserved in salt. Fermentation of garum took place outside in the dry heat. Garum may have been mixed with olive oil at the dinner table to make what we would now think of as a vinaigrette dressing.
Some of the Roman writers thought, erroneously, that garum is the liquid that is formed when fish rots, due to bacterial decomposition (Grainger, 2010). In fact, it is rather the result of fermentation, in which salt and the enzymes of the fish itself cause it to break down. For this process, the innards of the fish are very important, since the intestines contain large quantities of proteolytic enzymes that work break down the proteins in the fish. The fermentation process is accelerated by using salt that draws out the liquid from the fish and furthermore suppresses the growth of bacteria. Some authors believe that garum was not a primary product but a by-product from the salting of fish (Marzano, 2013).
It seems that there were local variations as to which types of fish and what parts of them were used and in what proportions. The sauce could contain large amounts of fish, especially if it was made from small anchovies, which almost liquefy under fermentation. It is possible that garum was made with blood and liquamen with whole fish. Even though the paste-like dregs, called allec, that were left behind when the liquid was skimmed off were malodorous, the garum itself appears to have had a mild, pleasant taste (Grocock and Grainger, 2006).
The garum trade reached its peak from the second to the fourth centuries CE, but garum never lost its appeal in the Mediterranean region, where it was used throughout the Middle Ages and Renaissance. It has survived to this day in the form of salted anchovies and anchovy paste, both of which have a cleaner taste because the innards are removed from the fish before they are processed. Some small companies in Italy and Spain have recently sprung up and are producing fish sauces based on ancient recipes for garum production.
Making a true garum is a slow process. But there is a shortcut, which survived from a Greek source about agriculture (Geoponica) compiled in the tenth century CE from even older works (Dalby, 2011).
In it there is a recipe for a sort of quick-and-easy garum for the benefit of “the busy Roman housewife.” This recipe calls for cooking salted fish and innards for about two hours. As it is made without any fermentation, it is milder than traditional garum and imparts much less umami.
We have produced such a fish sauce and analysed it in the present paper.
Even though there is evidence that the ancient Greeks and Romans used garum as a fish sauce more than 2500 years ago, we probably have to look to food cultures of the East, and especially to those in China, to find the origins of contemporary fish sauces. It was mostly the Chinese living in the coastal areas who made fish sauce, probably by combining small fish with the innards from larger cooking fish, causing fermentation.
Once soybeans were added to the salted and fermented fish in order to stretch the ingredients further, the liquid started to evolve into what would later become soy sauce, which came to be used much more widely, particularly inland.
The different varieties of fish sauces now made in Southeast Asia—for example, oyster sauce (which however is not a fermented product but an oyster extract)—all contain large quantities of glutamate and can be seen as counterparts to the Roman garum.
Interestingly, in contrast to such other taste additives as anchovy paste and certain original Asian fish extracts, fish sauce has no significant content of free 5′-ribonucleotides. In ancient Japan, a concentrated fish extract, irori or katsuo-irori, was produced by reducing to a paste the liquid in which bonito (a.k.a. skipjack tuna, in Japanese katsuo) had been cooked, using the same techniques as those now used to make katsuobushi. Katsuobushi is the component of classical Japanese dashi that contributes the 5′-ribonucleotides that interact synergistically with the glutamate from konbu to impart a strong umami taste. As katsuobushi dates back only to the 1600s, it is a much newer arrival on the culinary scene than irori.
Although irori disappeared from Japanese cuisine during the Meiji era (1867–1912) and is no longer on the market, a similar product, senji, is still made on the island of Kyushu in the southern part of Japan. When produced according to the traditional recipe, senji contains 900 mg of glutamate per 100 g and no less than 786 mg of inosinate per 100 g (Yoshida, 1998). Comparably high levels of inosinate are found in another traditional fish extract, rihakuru, from the Maldives. Both of these sauces are much richer in inosinate than katsuobushi, which has only about 474 mg per 100 g (Ninomiya, 1998).
Commercial fish sauces were purchased either in retail stores or directly from the producers.
Fresh mackerel (Scomber scombrus) was purchased from Albanifisk (Kerteminde, Denmark).
Wax moth larvae (Galleria mellonella) and grasshoppers (Locusta migratoria) were obtained from Avifauna, Krusaa, Denmark.
Pheasant (Phasianus colchicus) was obtained from a hunting friend of the Nordic Food Lab.
Split yellow peas (Pisum sativum) were obtained of the brand Møllerens (Valsemøllen, Esbjerg, Denmark).
Koji based on homegrown koji-kin, was originally obtained from a Japanese producer, but perpetuated at Nordic Food Lab since the fall of 2011.
Organic pearled barley for koji was obtained from Condi (Albertslund, Denmark).
Salt used for salting was either sea salt or ordinary household salt.
The acetonitrile was obtained from Fischer scientific (Roskilde, Denmark).
All other solvent and salts used for chemical analysis were from Sigma-Aldrich (St. Luis, MO).
The water used for chemical analysis was ultra-pure water obtained from a MilliQ-Integral 5 water purification unit (Merck-Millipore, Billerica, MA).
Preparation of barley koji proceeded by a protocol developed at Nordic Food Lab.
1. Soak pearled barley overnight.
2. The next morning, turn on koji chamber (upright fridge turned off and fitted with heating mat and temperature probe connected to PID controller) to 30 °C.
3. Steam barley (100 °C) for 90 min in a perforated gastro tray.
4. Remove barley from oven. While it cools, blitz koji-kin in thermomix to a fine powder (~20 g koji-kin /1 kg cooked barley).
5. Once the barley has cooled to a comfortable temperature (~60 °C), sanitize hands with alcohol, use don gloves, and break up chunks thoroughly. Pour or scoop the barley into standard full-size gastros to a depth of about 2 cm.
6. Prepare a warm, damp, wrung-out cloth to cover the koji.
7. When the barley has reached 35 °C, inoculate it with the powdered koji-kin and mix thoroughly to coat every grain. Smooth out the barley and cover it with the damp cloth.
8. Place the tray into the koji chamber (time T+0). Fill from the top racks down, to make use of the most heat. Place the temperature probe into the barley itself to keep track of the internal temperature; this is crucial to not letting it overheat. Put it in the koji on the uppermost shelf, as it is in the warmest part of the chamber. Close door.
9. At T+18 h, remove the tray and turn the koji over to aerate and redistribute spores. Redampen the cloth, cover, and return the tray to the chamber. Nestle the probe into the top shelf koji, and close the door.
10. At T+24 h, turn koji again. It should start to smell fruity and fragrant. This time, create two furrows in the koji lengthwise with hands; the furrows should be as deep as possible without being able to see the metal pan through the grains. Return the koji to the chamber with a newly dampened cloth. Nestle the probe into the top shelf koji. This time, keep the chamber door open a crack to prevent overheating.
11. At T+30 h, turn the koji a final time, again with furrows. Redampen the cloth and return the koji to the chamber. Nestle the probe into the top shelf koji with the door slightly cracked.
12. Monitor the temperature carefully over the next 18 h; it should stay as close to 30 °C as possible, and not much higher.
13. At T+36, the Aspergillus oryzae mycelia will have permeated the grains and they will hold together in a spongy white cake.
14. If using koji for further fermentation, use it while it is still white. If saving koji as koji-kin, let the Aspergillus oryzae continue to develop until it begins to sporulate and turn green. Once it turns green, break it up in the gastro and let it dry slowly, on top of the oven or somewhere warm and dry. Once it is completely dry, store it for future koji inoculation.
This recipe is adapted from an ancient Greek source about agriculture (Geoponics) compiled in the tenth century CE from even older works (Dalby, 2011).
2000 g very fresh mackerels
1 L water
400 g salt sprigs fresh oregano
1. Cut the whole mackerels, innards and all, into smaller pieces and put them in a pot together with the water, salt, and some oregano.
2. Bring the water to a boil, turn the heat to low, and allow the fish to simmer for about 2 h.
3. Strain out the liquid, first in a sieve or colander to remove the large pieces and then through cheesecloth to ensure that the liquid is completely clear.
4. Store the finished sauce in the refrigerator in a bottle with a stopper
Three different approaches, B1, B2, and B3, have been used, basically using different parts of fresh mackerel as a source. B3 furthermore uses a barley koji for enzymatic fermentation. B4 was originally an experimental fish sauce and is now available as a commercial product with the trade name Flor-de-Garum.
For the sake of completeness we provide two versions of the recipe. One that we have used in the laboratory under controlled conditions and another one (kitchen version) that requires warm outside summer conditions.
3 kg very fresh mackerels (one whole mackerel and head, bones, entrails of four mackerel)
450 g salt (15% salt)
Lab version.
1. Cut the whole mackerels, innards and all, into smaller pieces and place them in a container.
2. Mix in the salt.
3. Cover the container with a loose-fitting lid and place it in a thermostat cabinet at 28 °C for several months. For purpose of analysis, samples have been drawn from the container with certain intervals and subsequently been sterilized and stored at −20 °C before analysis. Before analysis, the samples are thawed and filtered.
Kitchen version (Mouritsen and Styrbæk, 2014).
1. Cut the whole mackerels, innards and all, into smaller pieces and place them in a crock.
2. Mix in the salt, cover the crock with a loose-fitting lid, and place it outside where it is warm, preferably in the sun.
3. Allow the fish to ferment for a couple of months, turning the fish pieces in the salt once in a while.
4. Strain out the liquid, first in a sieve or colander to remove the large pieces and then through cheesecloth to ensure that the liquid is completely clear.
5. Store the finished sauce in the refrigerator in a bottle with a stopper.
For this recipe, one can also use just the entrails and blood from mackerels as in B2.
Same as the kitchen version B1 based on mackerel, but with the use of only blood, gills, and entrails.
The sample analysed was kindly prepared by Søren Mørch.
The procedure to produce fish sauce using koji is the same as described below for insects, yellow peas, and pheasant.
This garum is an experimental product provided by Jacob Sørensen (Thyborøn, Denmark). It is based on whole, very freshly caught smelt in a mixture with 25% salt. The sauce develops over a period of 12 months (B4) and 18 months (B5) at a temperature of 27 °C.
This garum was developed based on an ancient Pompeii recipe using anchovies, first as an experimental product and now available as a commercial product with label Flor-de-Garum (Vargas et al., 2014).
The analysis was performed on the experimental version.
At Nordic Food Lab we have adopted and adapted central parts of the traditional procedure for making soy sauce for other fermentation purposes in order to obtain umami-rich sauces (Evans, 2013). The recipes for different sauces follow the same general process, with some minor modifications between different raw materials. The agent of transformation used for the fermentation is Aspergillus oryzae that is grown using pearl barley as substrate. Cooked pearl barley is inoculated with our house culture of koji (vegetative Aspergillus oryzae moulds) that has been cultured for more than 6 years. The inoculated barley is kept at around 30 °C for 30–36 h, at which point the mold will have completely overgrown the barley. Precise instructions are given above.
The active koji is subsequently mixed with various protein-containing ingredients and salt to a level higher than 10% salt in the finished solution.
The general procedure adopted for making garums using koji is the same for fish, yellow peas, and pheasant.
1000 g raw material
300 g water
240 g salt
250 g barley koji
1. Blend the raw materials with water and salt until it is broken up as a puree but not smooth.
2. Mix the blend with the koji.
3. Place the mixture in glass containers with cling film over surface in order to keep out oxygen.
4. Incubate at 40 °C for at least 10 weeks.
5. Taste the sauce. If deemed ready, filter it through filter paper in a chinois, bottle the liquid, and pasteurize it at 72 °C for 15 s
For pheasant garum, use 500g of barley koji instead of 250g. For yellow peas, boil the peas until tender and cool down before mixing with koji. Extra virgin grasshopper garum is the first fractioning of the broken down garum that is produced by only using gravity to separate the solid parts from the sauce.
The paste remaining from drainage of the fermented pea sauce is a product related to miso, and has hence been called peaso.
Physico-chemical analysis
The total osmolarity was measured on a Gonotec osmomat 030 (Gonotec GmbH, Berlin). Samples were diluted as required with ultrapure water before analysis. The pH was measured on the un-treated samples with an Eutech pH510 pH-meter (Eutech Instruments Pte Ltd, Singapore) equipped with a VWR Universal pH electrode (VWR, Herlev, Danmark).
Amino acid analysis
Detection and quantification of free amino acids were performed by HPLC-MS using a Shimadzu LCMS 2020 MS equipped with an electrospray interface (ESI). The HPLC consisted of a DGU20-A5 inline degasser, two LC-20 AD pumps, a high-pressure mixer, an SIL20AHT autosampler, a CTO 10 Column oven, and an SPD-20A UV detector, all from Shimadzu (Holm & Halby Brøndby, Denmark).
Separation of the 17 different free amino acids was performed on an Imtak Intrada amino acid column (Imtakt Corporation, Kyoto, Japan) by gradient of acetonitrile with 0.1% formic acid and 100 mM NH4HCO2, in 21 min. The gradient consisted of first 3 min with 14% NH4HCO2 followed by an increase of the NH4HCO2 to 100% during the next 7 min. This level was maintained for 2 min, whereafter it was returned to 14% in 2 min and then allowed to re-equilibrate for the rest of the time.
Individual AAs were detected by selected ion monitoring (SIM) at appropriate m/Z values and were quantified by comparison with a standard curve prepared from commercial an available amino acid standard from Sigma-Aldrich. All samples were diluted 50 times with 0.1 M HCl and filtered through a 0.2 μm PTFE syringe filter before analysis. Due to their similar mass, Ile and Leu are difficult to separate in some samples and we have therefore for convenience only measured their sum. Cystine is measured as a dimer Cys2 but all data reported labelled by Cys2 have been renormalized to monomer concentration.
Nucleotide analysis
The four different free nucleotides, AMP, IMP, GMP, and UMP, were measured by HPLC-MS. The used LMS was the same as used for amino acids analysis but the analytes were separated on a Waters XBridge amide 100 mm x 3 mm HILIC column (Waters, Hedehusene, Denmark) by a gradient of acetonitrile and 10 mM ammonium formate at pH=3. The gradient consisted of first 2.5 min with 15% 10 mM ammonium formate, followed by an increase to 70% 10 mM ammonium formate during the next 20 min. This level was maintained for 2 min and subsequently lowered to 15% again in the next 5 min, and then kept at that value for 5 min. The nucleotides were detected in positive mode at appropriate m/Z values and quantified by comparison with standard curves obtained from the pure nucleotides. All samples were diluted 4 times with phosphate buffer (50 mM, pH=7.2) and filtered through a 0.2 μm PTFE syringe filter before analysis.
Total protein
Total protein was measured by a Bradford assay (Bradford, 1976).
The Bradford reagent was obtained from Sigma-Aldrich and the assay was performed as described by the manufacturer by mixing the appropriate amount of sample and Bradford reagent in a 96 well plate flowed by measuring the absorbance at 595 nm. Absorbance was measured in a BMG Fluostar plate reader (BMG Labtech, Ortenberg, Germany). All samples were diluted as needed with ultra-pure water before analysis.
Sodium and potassium analysis
Sodium and potassium contents were determined by flame AAS on a Shimadzu AA7000 spectrometer equipped with an ASC-7000 autosampler (Holm & Halby Brøndby, Denmark). Samples were diluted 1000 times with 1 M HNO3 of trace-metal grade (Normatom, VWR) before analysis.
The samples were analysed in the so-called microsampling method, by which 60 μL of the sample is injected into the flame and then quantified by obtaining the area of the obtained absorbance peak. The concentrations were calculated from at standard curve obtained from the analysis of a commercial standard (Traceselect, Sigma Aldrich).
Chloride analysis
Chloride concentrations were determined by a miniaturized mercury thiocyanate- Iron(III) nitrate assay in a 96 well half area microplate (Greiner Bio One, Frickenhausen, Germany) as described by Merchant (2009). In short, a solution of Hg(SCN) in methanol is mixed with an aqueous solution of Fe(NO3)3 and the sample in an appropriate ratio. The concentration of chloride is then determined from the absorption of the formed iron thiocyanate complex. Absorption values were obtained at 408 nm in a BMG Fluostar plate reader. Note that this method has a rather large uncertainty due to the low volume method that was chosen for safety reasons.
Sensory evaluation
Initially, potentially panellists were screened for their sensitivity to monosodium glutamate (ISO: 3972:2011, 2011). The purpose was to ascertain that they could perceive the compound, and understand the sensory descriptor. The training consisted of two stages. The first stage was a descriptor-development session (2 h). In the descriptor-development session, the panellists came up with all the descriptors they could think of to describe the products. The panel leader chose the descriptors to use for the training based on consensus among the panellists. In the second stage (3 sessions of 1ó−2 h) the panellists were trained for the assessment of the fish sauces. In each session a subset of the samples presented in the evaluations were used. Fourteen descriptors were chosen for the final evaluation; see Table 4. These included descriptors for aroma, flavour, mouthfeel, and taste.
The descriptive sensory study was conducted with 9 paid external panellists in four sessions. In each session, 12 or 13 samples were served (i.e., a total of three sensory replicates). The training and the sensory evaluations were carried out in a sensory facility, with panellists seated at individual tables and not disturbing each other during tastings.
Statistical analysis of sensory data
Data from the descriptive analysis was analysed by analysis of variance (ANOVA) and multivariate data analysis (ANOVA–partial least squares regression (A–PLSR)). Mixed model ANOVA for individual descriptors was performed with products (n=17) as fixed factors and panellists (n=9) as random factors. This method is commonly applied to data from descriptive analysis (Naes et al., 1998). ANOVA– PLSR (A-PLSR) is a multivariate regression method where the effect of design factors on the response variables (here, the chemical and sensory descriptors, respectively) is evaluated (Martens and Næs, 1989; Martens and Martens, 2001). The method avoids multi-collinearity problems by modelling latent variables representing the main variance common for the variables. The method evaluates effects of the experimental design variables on chemical characterisations and sensory properties. Here it is used as a graphical alternative to ANOVA. Mean ratings over panellists from each replicate were used for A–PLSR. For multivariate analyses, cross-validation was performed, leaving out one sensory replicate at a time (Martens and Næs, 1989). Jack-knifing with replicates served as the validation tool for all multivariate analysis, comparing the perturbed model parameter estimates from cross-validation with the estimates for the full model (Martens and Martens, 2000).
The relationship between chemical characteristics and sensory properties of the fish sauces is analysed by PLSR regression carried out on mean data. For chemical measurements mean data are means over replicates of measurements (3–4 replicates unless otherwise stated). For sensory data mean, data are means over panellists (N=9) and replicates (n=3).

Fig. 1. Traditional garum made from whole fresh mackerel. (a) Freshly cut whole mackerel mixed with salt. (b) Fermented mackerel after 3 months at 28 °C. (c) Filtered garum sample from the fermentation experiment in (b).
An illustration of stages of the production of an experimental fish sauce based on fresh mackerel is shown in Fig. 1.
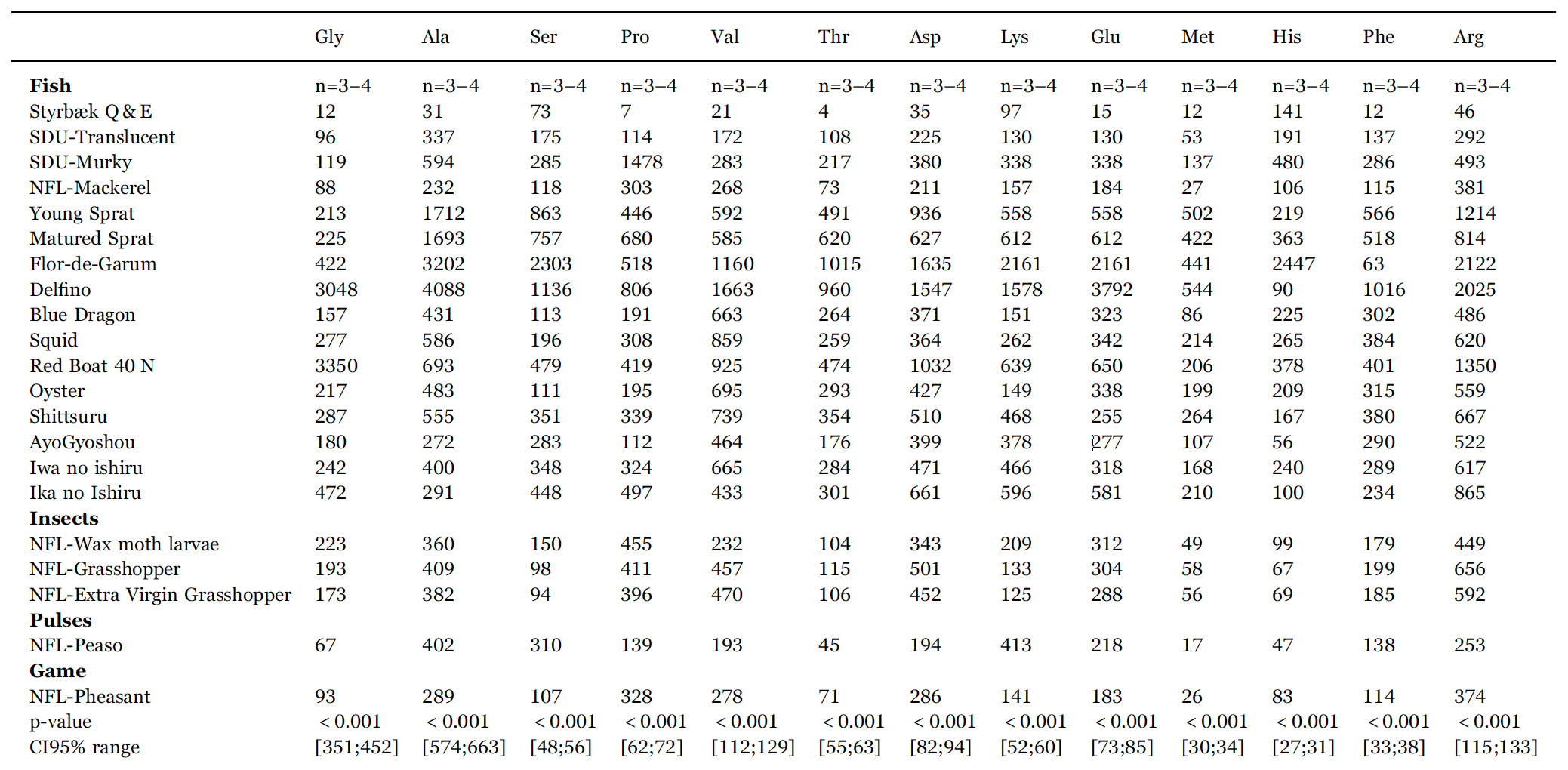
The results of the chemical analysis of the different fish sauces are presented in Table 2 (free amino acids and total protein) and Table 3 (Na, K, Cl, pH, and osmolarity). No data are presented from the nucleotide analysis since for none of the fish sauces are we able to detect any amount of free nucleotides (AMP, GMP, IMP, UMP) within the detection limit (0.0005 mg/100 mL).
In order later to evaluate the umami potential, we have in Table 1 listed the Glu contents in groups of fish, insects, pulses, and game. It can be difficult to compare numbers for the different products not knowing the details of the way they are prepared, in particular whether the commercial sauces have been adjusted in taste by dilution by water.
However, it is clear that they all, except the quick-and-easy non fermented garum, contain substantial amounts of free glutamate. From det data for the experimental fish sauces B4 and B5 we observe, that the fish sauces with the longer fermentation periods contain more glutamate. The two most traditional European garums, Flor-de-Garum and Delfino, made respectively on anchovies and sardines, are clear outliers with very high levels of glutamate.
It also appears that garums made on anchovies, sardines, and sprats contain more glutamate than those produced from mackerel. Moreover, the mackerel garum made on blood, gill, and entrails have more glutamate than those based on whole fish.
The fermented sauces made on insects, yellow peas, and pheasant have glutamate levels compared to those fish sauces with the smallest levels.
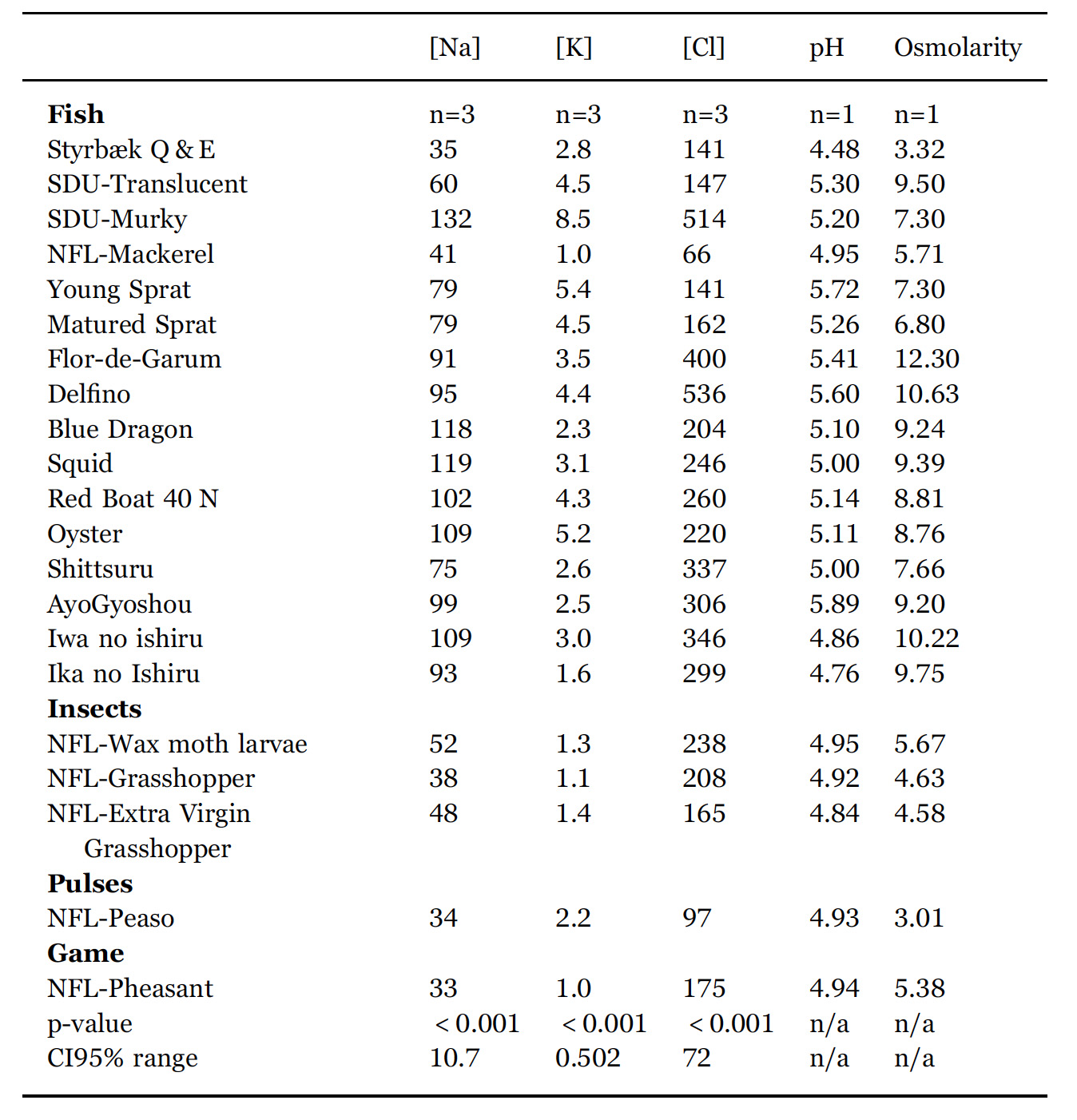
The level of acidity for the sauces listed in Table 3 lies typically in the range 4.5 < pH < 5.5 which is similar to what is found in other studies of fish sauces (McIver et al., 1982). The salt concentration is dominated by sodium.
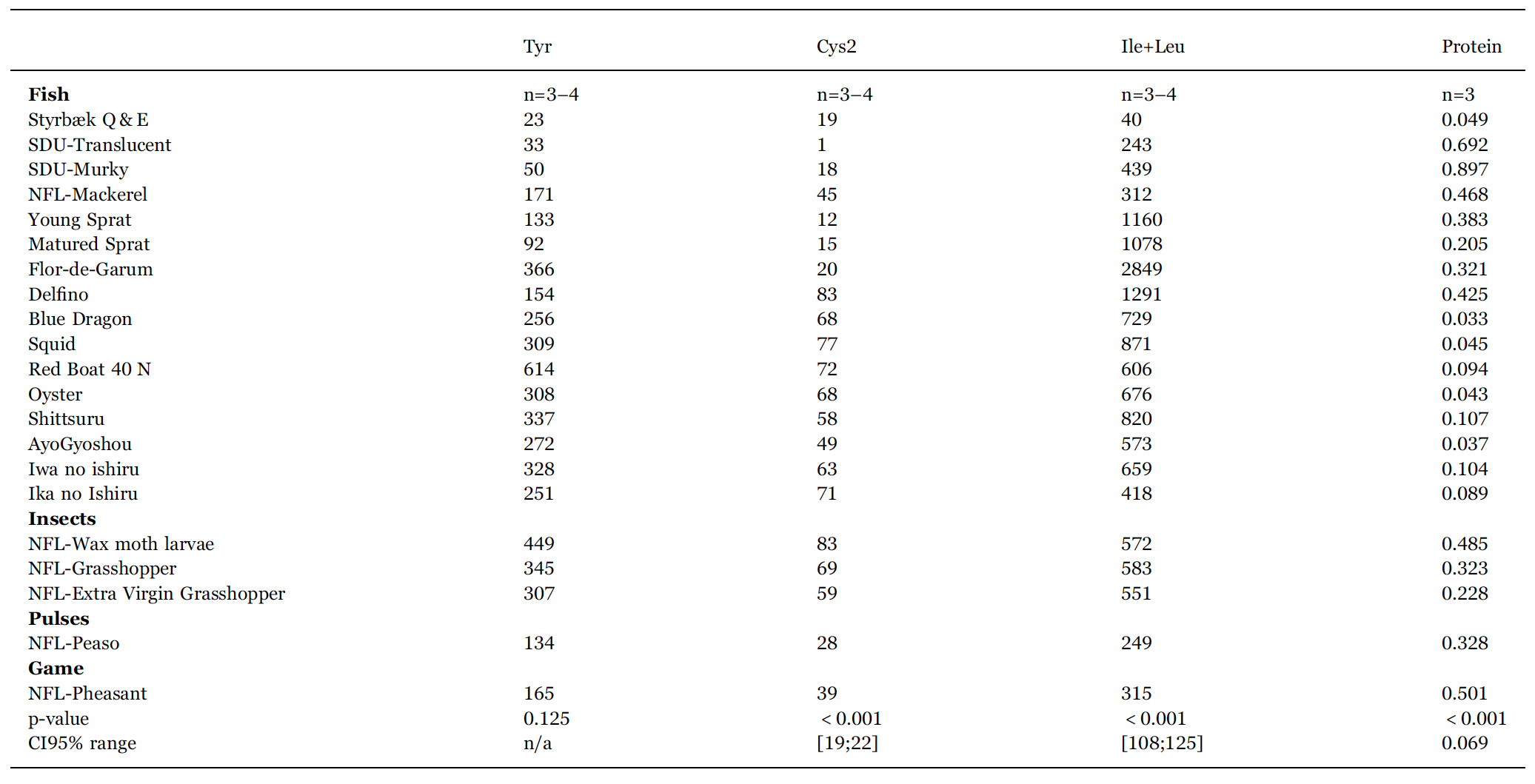
In order to clarify whether the different fish sauces are statistically different from the point of view of their combined chemical and physic chemical characteristics, an ANOVA analysis of the data in Tables 2, 3 is carried out. In the bottom of the two tables p-values and CI95% ranges show that all variables except tyrosine concentration contributed to separate the samples statistically. Therefore we can conclude that each sauce is unique in the sense it is statistically significantly different from all the other sauces investigated.
We have carried out an A-PLSR in order to obtain a lower dimensional representation of the main differences in the different sauces. Fig. 2 gives a graphical representation of the first two dimensions of this analysis, showing the largest (dimension 1, 49% of the total variance) and second-largest (dimension 2, 10% of the total variance) part of the variance in the physico-chemical data. It is a correlation loading bi-plot, showing both the distribution of the sauces and the interrelationship between the chemical variables. From here an overview of the interrelationships between the samples is obtained.
Clustering of similar sauces can be observed. The NFL-family using koji in the fermentation forms such a cluster. The two traditional European garums, Flor-de-Garum and Delfino, are very different from most of the other sauces. Going from left to right in the bi-plot, there is increasing concentration of Na, Cl, and amino acids. His appears in the lower part and Cys2 in the upper part of bi-plot. Sauces with high protein content are found in the lower part and appear to be uncorrelated to the amino acid concentration.
List of fermented products investigated. 1–3 samples of each product were subjected to physico-chemical analysis.
a Mackerel (Scomber scombrus), sprat (Sprattus sprattus) anchovy (Engraulis encrasicolus), sandfish (Arctoscopus japonicus), sweetfish (Plecoglossus altivelis), sardine (Sardina pilchardus), squid (species not declared by producer), wax moth (Galleria mellonella), grasshopper (Locusta migratoria) split yellow peas (Pisum sativum), and pheasant (Phasianus colchicus).
Contents of free amino acids (in units mg/100 mL) and total protein (in units g/L) in the fermented sauces listed in Table 1. n is the number of samples entering the mean value quoted. p-values and 95% confidence intervals (CI95%) are from ANOVA. Confidence intervals vary, as some samples are analysed in triplicates and other in quadruplicates. Ala: alanine; Arg: arginine; Asn: asparagine; Asp: aspartic acid (aspartate); Cys: cysteine, Gln: glutamine; Glu: glutamic acid (glutamate); Gly: glycine; His: histidine; Ile+Leu: isoleucine+leucine; Lys: lysine; Met: methionine; Phe: phenylalanine; Pro: proline; Ser: serine; Thr: threonine; Trp: tryptophan; Tyr: tyrosine; Val: valine. Due to their similar mass, Ile and Leu are difficult to separate in some samples and we have therefore for convenience presented their sum.
The mean ratings for all descriptors for 17 selected sauces for sensory analysis are listed in Table 4. Among the 14 descriptors, two did not contribute to separate the samples (sour and artificial chemical), one more had a p-value of 0.016 (yeasty), whereas the remaining 11 have p-values of 0.001 or less. The results in Table 4 thus show that the sensory analysis is precise enough to discriminate all fish sauces from one another, i.e., each fish sauce has a unique sensory profile.
The results from the multivariate analysis (A-PLSR) is visualized in Fig. 3 that is a correlation loading bi-plot, showing both the distribution of the fish sauces and the interrelationship between the descriptors.
The PLSR-analysis demonstrates that two underlying dimensions, describing 56% of the total variance in the data averaged over panellists, are sufficient to explain the systematic part of the variation in the sensory properties.
A pattern emerges from the results. Styrbæk Quick-and-Easy (Q&E) garum is located by itself in the upper left part of the plot. It is characterized by having the lowest intensity of umami (cf. Table 4) and is thus positioned inversely to umami. In the left part of the figure, the negative part of dimension 1, the four NFL-garums from Nordic Food Lab are positioned.
Along with Styrbæk Q&E, they are the samples lowest in salt taste and after taste. Those descriptors are located as the most extreme in the positive part of dimension one. In addition, the samples in the left part appear to be more bitter, but a close inspection of Table 4 reveals that it is only NFLPheasant that has a significantly higher bitterness.
Further scrutiny of the multivariate analysis reveals that the third underlying dimension (not shown) almost exclusive separates NFLPheasant from all other samples, and particularly so regarding the descriptors bitter and caramelized. In the upper right part are the two SDU-garums, translucent and murky. The two samples are the most fishy sauces (cf. Table 4). The two remaining sauces that were produced in Denmark are Matured and Young Sprat. They are located closer towards the centre of the plot, but still with a relatively high intensity of fishy taste. The southern European fish sauce Delfino, and the East-Asian fish sauces are characterized by high saltiness, umami taste, and more cheesy. Particularly Oyster, Squid and Red Boat have a high intensity of cheesy taste. All in all, the sensory analysis shows a clear grouping of the garums according to the production processes, more so than the raw material (fish, molluscs, insects, etc).
pH, osmolarity (mol/kg), sodium (g/L), potassium (g/L), and chloride (g/L) content of the fermented products listed in Table 1. n is the number of samples entering the mean value quoted. p-values and 95% confidence intervals (CI95%) are from ANOVA. Confidence intervals vary, as some samples are analysed in triplicates and other in quadruplicates. For pH and osmolarity no statistical analysis is made as they are obtained by single measurements.
In Fig. 4 is shown a correlation loading bi-plot for dimensions 1 and 2 derived from a PLSR analysis of the relationship between chemical characterisations and sensory analysis. This plot looses some systematic information compared to correlation plots involving chemical and sensory information separately, cf. Fig. 2 and Fig. 3, respectively. It indicates that not all sensory differences are captured by the chemical characterization. It is possible that additional data for aroma compounds obtained by gas chromatography may have improved this situation (Lapsongphon et al., 2015).
Some interesting observations can be made from Fig. 4. The concentration of His and a fishy taste appears to be correlated. Na and Cl are good predictors of salty taste and after taste. Glu and Asp are, maybe surprisingly, not particularly good predictors of umami taste and after taste.
Fig. 2. Correlation loading bi-plot for dimension 1 and 2 derived from an A-PLSR analysis of chemical and physico-chemical descriptors. The inner and outer circles represent 50% and 100% explained variance, respectively, in the underlying dimensions. For sample identities, see Table 1. This common map of samples and chemical variables demonstrate the interrelationship and differences between the different sauces.
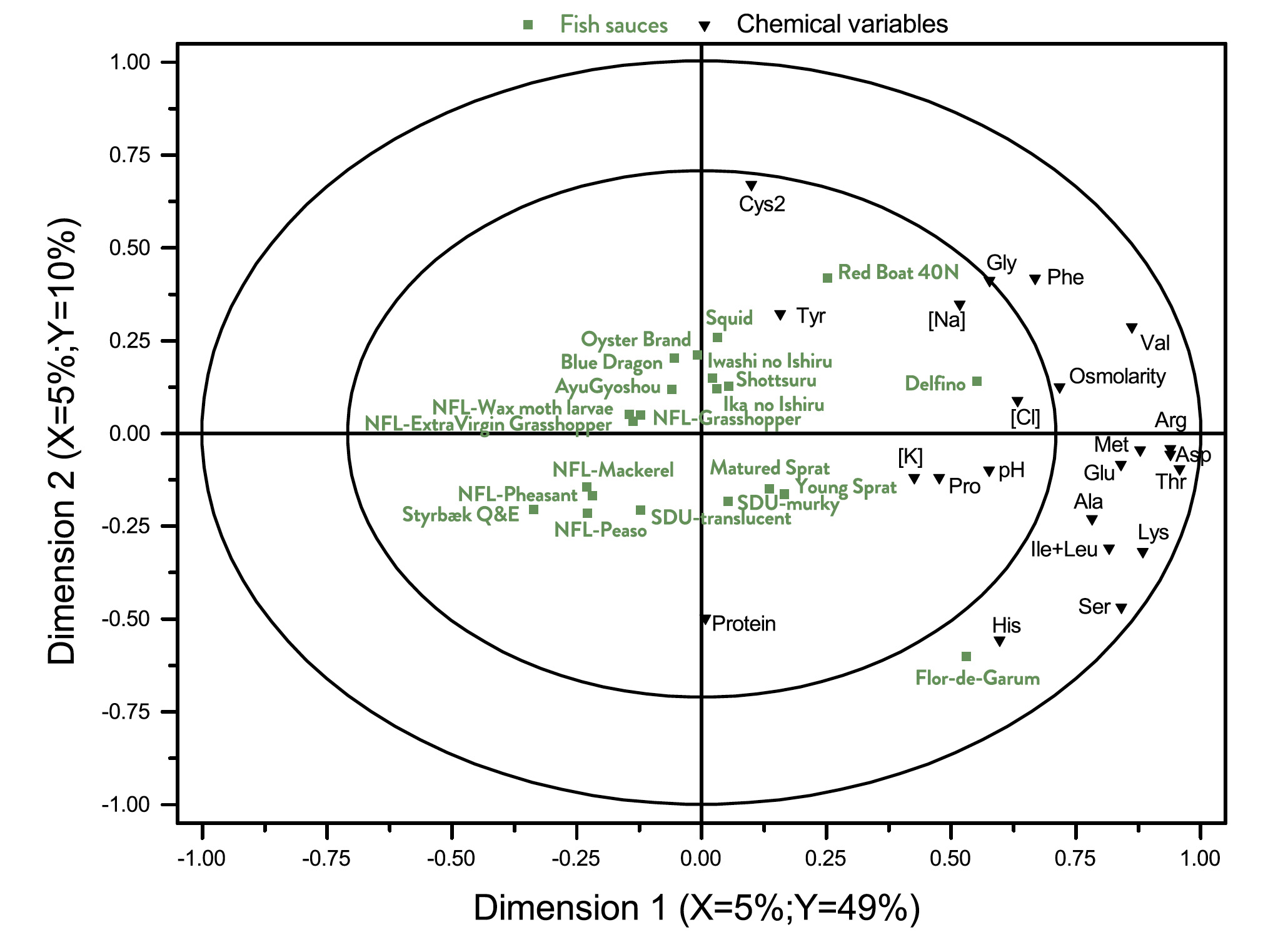
Fig. 3. Correlation loading bi-plot for dimension 1 and 2 derived from an A-PLSR analysis of sensory variables. See Table 1 for sample identities. The sensory variables are listed in Table 4. This common map of samples and sensory variables demonstrate the interrelationship and differences between the different sauces.
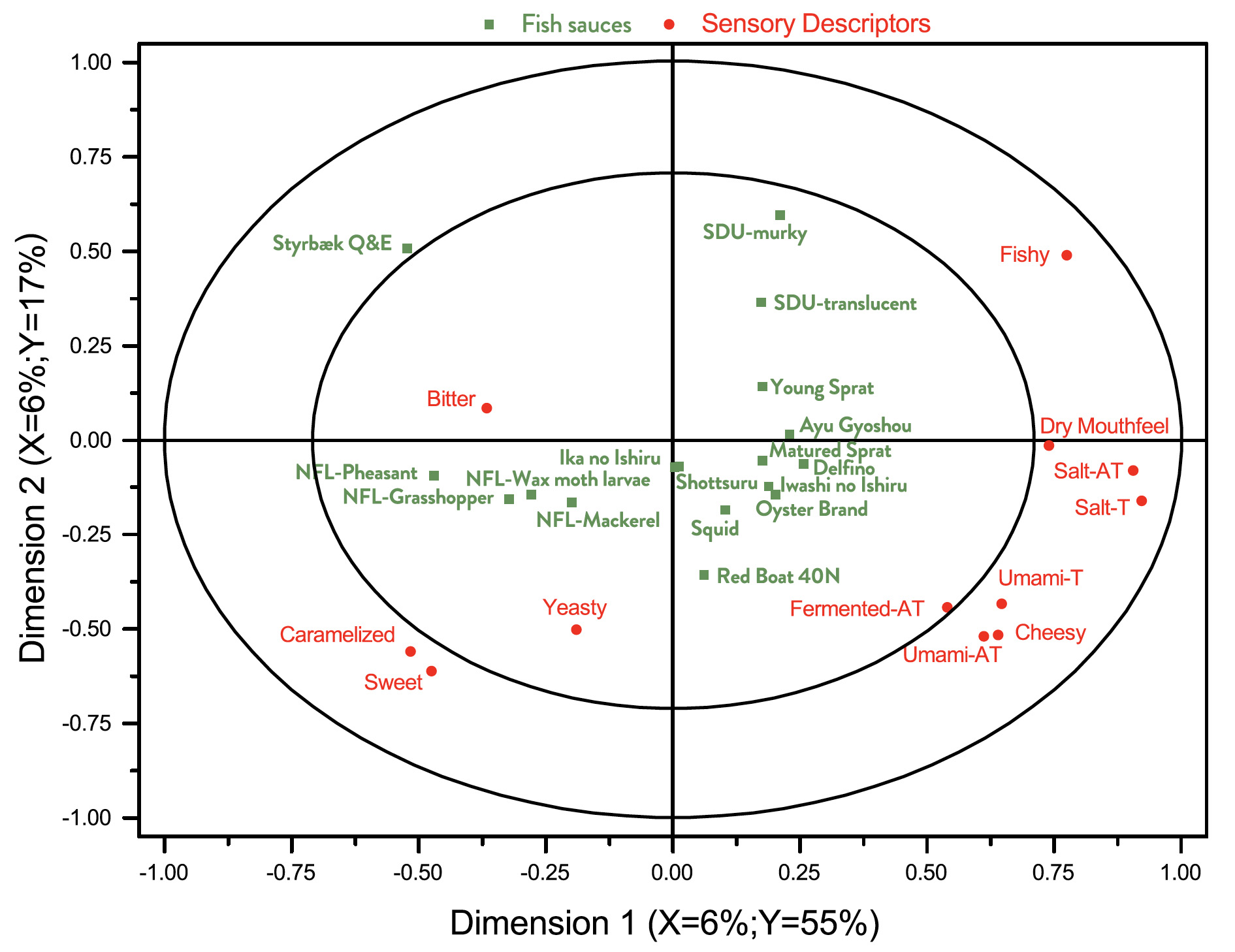
Fig. 4. Correlation loading bi-plot for dimensions 1 and 2 derived from a PLSR analysis of the relationship between chemical characterisations and sensory analysis. See Table 1 and Table 4 for sample identities and sensory variables, respectively.
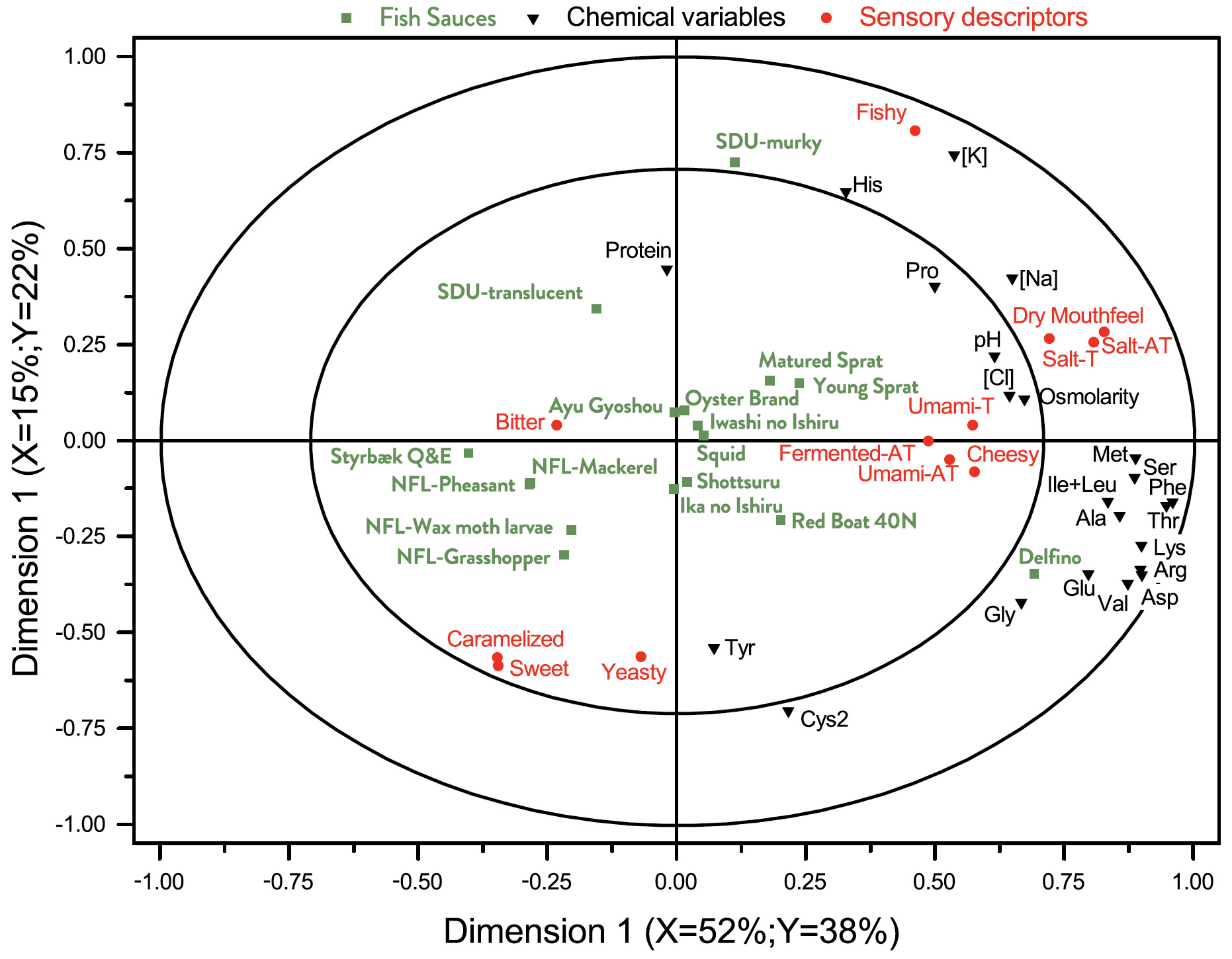
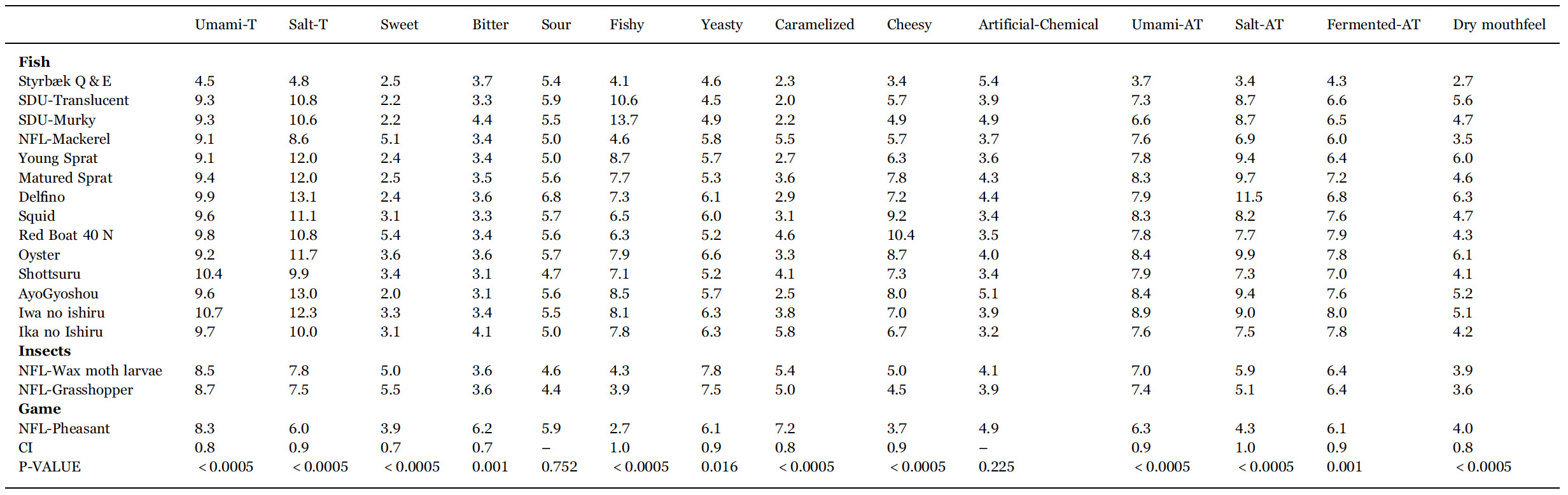
Table 4
Mean rating (over panellists and replicates) of intensity for all sensory descriptors for the 17 samples subjected to descriptive analysis. p-values and 95% confidence intervals (CI95%) are from ANOVA. Labels T and AT refer to ‘taste’ and ‘aftertaste’, respectively.
We have in the present paper performed a comparative study of a range of fermented sauces based on fish, molluscs, insects, pulses, and peas. The range of sauces encompasses both experimental sauces from our own laboratories as well from others. In addition we have investigated a range of commercial fish sauces. The purpose of the study was to characterise the different sauces by chemical, physicochemical, as well as sensory evaluation and uncover possible relationships between production methods and materials, chemical composition, and sensory characteristics.
Fermented sauces are commonly used as condiments in cuisines across the world, in particular with respect to add salty and umami taste to foodstuff. Most sauces available commercially are derived from a variety of fish, molluscs, and beans (in particular soy beans) but there is in principle no reason not also to consider producing fermented sauces from other raw materials with high contents of proteins, such as insects, pulses, and meat. In the present paper we have performed the first analyses of fermented sauces based on insects, and we have in addition studied fermented sauces from yellow peas and pheasant. We have found that these novel sauces have some superior sensory qualities and should be further considered for a wider use not only in the innovative kitchen, but possibly also on a commercial basis.
The quality, and with it the content of substances that impart umami, of all of these fermented sauces is very dependent on the raw ingredients that go into them and the method of production. Many of the commercially available products contain a great deal of salt and added MSG, and they do not live up to the high standard that is the hallmark of traditionally made fish sauces.
We have found, that all fermented sauces contain high levels of free amino acids, particularly Glu and Asp that are known to elicit umami taste. In contract, none of the sauces contain any measurable trace of free nucleotides. Hence, there is no basis for umami synergy in any of the sauces. This is also the case for those sauces where the fermentation has also involved the use of a koji mold. This finding is consistent with results of a previous study that did not find free nucleotides in fermented mackerel using soy sauce koji (Funatsu et al., 2000a, 2000b). As is well known from katsuobushi production special attention must be paid to the early stages of the production such that inosinate is not converted to inosine. Moreover, it is well-known that the inosinate produced in the dead fish from muscular ATP over time decays into inosine after some days (Howgate, 2006).
Use of koji in production of the NFL-garums appears to have placed all these sauces, it be produced by fish, insects, pulses, or game, in a special group with low salt and good flavour characteristics. Earlier work on fermented fish sauces based on mackerel (Funatsu et al., 2000a; 2000b) and salmon (Takeshima et al., 2001) also found that koji imparted a special flavour to the sauces.
There are a number of issues we have not dealt with in the present paper. One is the aspect of microbiology which we have circumvented by always using high amounts of salt in the experimental sauces in addition to reasonably low values of pH. Another issue is the content of histidin variation during fermentation (Taira et al., 2007), which is important because histidin can be converted to histamine that can provoke allergic reactions that can be a problem with some fish sauces with very high levels of histidin and histamine. By using extremely fresh fish for fermentation this problem can to some extent be alleviated.
One of the more surprising outcomes of the present study is that, although the sauces are generally high in free glutamate, umami synergy is not expected to play any significant role for the flavour of these fish, insect, game, and pea sauces. The sensory analysis shows a fairly good prediction of sensory properties from the chemical characterization of the sauces. However, the relationship between glutamate/ aspartate concentration and intensity of umami taste is not simple. It demonstrates that in the complex solutions that constitute these sauces, there may be other perceptions that interfere with the main umami-tasting compounds.
In the present paper we analyse the chemical composition of various fermented products, both commercial and experimental ones, with a view to relate their chemical composition to umami taste. We describe simple procedures for preparing fish sauce from mackerel, both fast cooking techniques and long-lasting fermentation techniques inspired by traditional garum production in the antiqueue Roman period as well as by use of ancient Japanese fungal inoculation methods. We extend these techniques to fermentation of insects, like moth and grasshoppers, pulses like yellow peas, and game like pheasant. Finally, we deliver a sensory evaluation of the various fermented products and make an assessment of their umami potential.
Regarding the potential umami flavour of the fermented sauces, our finding is, that whereas the various sauces contain substantial levels of free glutamate that elicits basic umami we find no trace of free nucleotides that could provide for umami synergy.
This work was supported in part by a grant to Smag for Livet (Taste for Life) from Nordea-fonden (both to University of Southern Denmark and Nordic Food Lab at University of Copenhagen). The HPLC-MS equipment was supported by a grant from the VILLUM Foundation, and the AAS was acquired via a grant from the Carlsberg Foundation.
Cuong T. Pham is thanked for kindly providing free samples of Red Boat Fish Sauce. Josh Evans is thanked for transforming Nordic Food Lab ׳s garum recipes into a format suitable for this publication. Klavs Styrbæk has provided the quick-and-easy garum. Poul Rasmussen has kindly supplied the fresh mackerel used for our garum experiments.
Søren Mørch is gratefully acknowledged for providing us with a sample of his homemade garum from mackerel gills and blood. Mio Kuriwaki and Kumiko Ninomiya from the Umami Information Center are thanked for sending us free samples of different Japanese fish sauces.
Fini Sánchez García kindly provided the experimental Flor-de-Garum from Cádiz based on a recipe from Pompeii. Jacob Sørensen (Montenegro Sea Food, Thyborøn) has kindly supplied a sample of fish sauce made with young sprats. Koji Kinoshita is thanked for help with Japanese language issues.
Information
The SELO DE MAR website uses cookies to give you a better online experience. By continuing your visit to our website, you agree to the use of cookies.
Contact
hello@selodemar.com